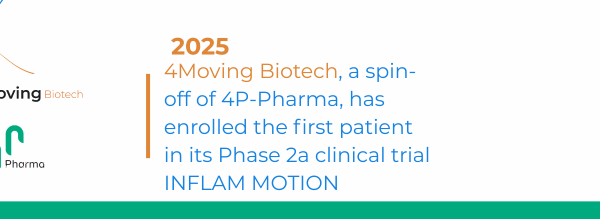
4Living Biotech, a biotechnology company and subsidiary of 4P-Pharma, specializing in the treatment of respiratory infections, has received approval from the French National Agency for Medicinal Products (ANSM) and two other European agencies to initiate a Phase IIb clinical trial of LEONARDO in patients with acute respiratory failure associated with COVID-19.

The government financed this project as part of the “Investissements d’avenir” program.